Law of the conservation of mass
the mass of the products (overall) always equals the mass of the reacting substances
A Graphic of the Atom
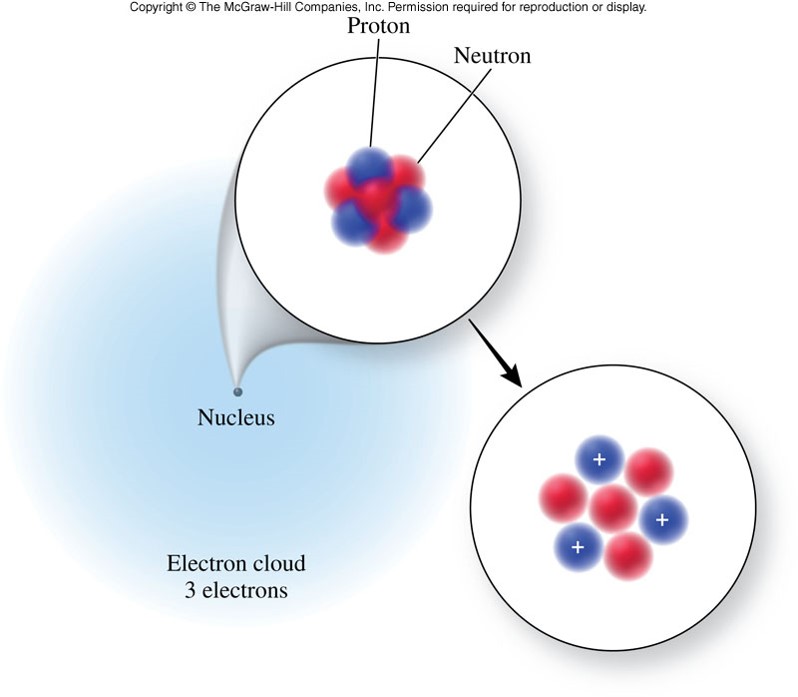
Atomic number and Mass Number
- Atomic Number (Z)
- the number of protons in the nucleus of an element's atom
- is generally found on the periodic table above the elemental symbol
- Mass Number (A)
-the number of protons and neutrons in the nucleus of an element's atom
is generally found below the elemental symbol on the periodic table - Neutron Number (N)
- the number of neutrons in the nucleus of an element's atom
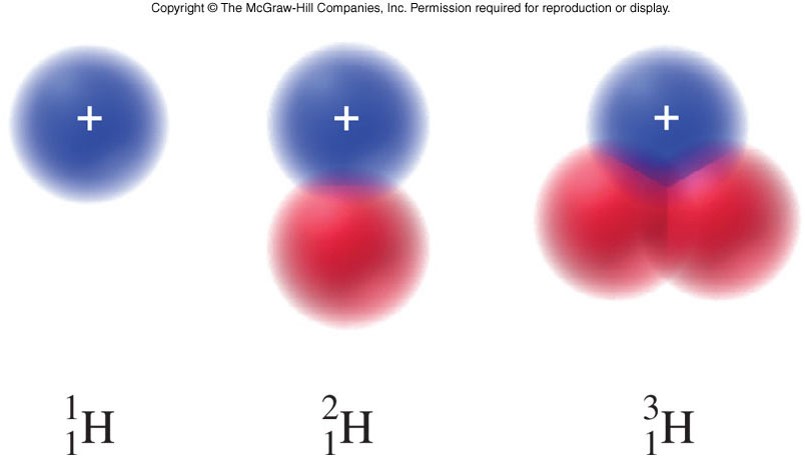
An isotope of an element is an atom that contains a specific number of neutrons.
Isotopes, Hydrogen, Deuterium, and Tritium
An atomic mass unit (amu) is equal to 1/12 the mass of a carbon-12 atom.